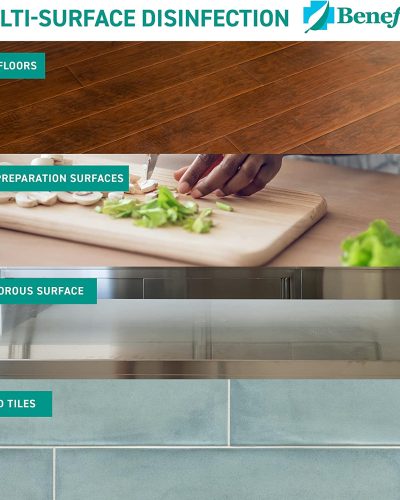
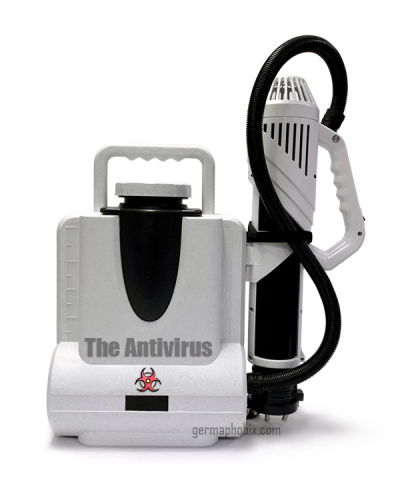
Sanitization, Disinfection and Cleaning Supplies
April 10, 2020 2024-01-24 1:55Sanitization, Disinfection and Cleaning Supplies

Like a Pro









Electrostatic Sprayer

Last Chance Deals
End in:
Sold out
AirSafti Bio-Static Vehicle Disinfectant Generator
4.64
(28)
$189.99$299.99

Lightning Shipping
Sale !
Benefect Botanical Decon 30 Disinfectant Cleaner
4.50
(12)
$69.99-$6,999.99$109.99-$8,499.95

Lightning Shipping
+5 different sizes
22% off!
13W Single Lamp In-Duct UV Light HVAC Air Purifier
4.79
(24)
$69.99$89.99

Lightning Shipping
20% off!
Fehom 130 Pints Commercial Dehumidifier (6,000 Sq. Ft)
4.50
(50)
$569.99$709.99

Lightning Shipping
Best Sellers
From our Blog
Great quality!
This is a great design and I hope that we will create a website with a good signature. ThemeMove team is reactive and kind. Thanks for the help so far.
Luke Olfert
/ Web Developer, CanadaMy luck
Before my purchase, I had a look of many other themes & clubs that are available out there. So, ThemeMove is just good luck for me. 5-star rating deserved.
Ryan Teixeira
/ Designer, UKNo coding Require
Very prompt, and fixed the problem within a day of submitting a ticket. I just started to use WordPress, and I absolutely love ThemeMove's themes
Daniel Pereira
/ Marketer, FranceGood customer service
Theme has nice and rich features, also good design. This is our first time with Developer - ThemeMove
and so far everything is great.
Emily Noth
/ Agency, GermanyLove this
Pretty much every single pixel can easily be customized. Great theme!
Karoline Smith
/ Video Producer, ItalyFree Shipping
Most items shipped free with 3 business days delivery
Office Hours
Mon-Fri :
9AM-6PM EST
Sat & Sun:
10AM-4PM EST
9AM-6PM EST
Sat & Sun:
10AM-4PM EST